 Home
Home
 Back
Back
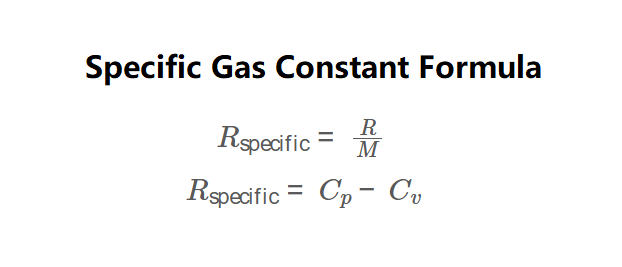
Definition: This calculator computes the specific gas constant (\( R_{\text{specific}} \)) for a gas or mixture, used in thermodynamic equations like the ideal gas law (\( P = \rho R_{\text{specific}} T \)). It supports two methods, as shown in the formula image above:
Purpose: Essential for engineers, scientists, and students in thermodynamics, aerodynamics, and chemical engineering to analyze gas behavior.
The specific gas constant is calculated using one of two formulas, as depicted in the formula image:
\( R_{\text{specific}} = \frac{R}{M} \quad \text{or} \quad R_{\text{specific}} = C_p - C_v \)
Where:
Available Gases and Molar Masses:
| Gas | Molar Mass (g/mol) |
|---|---|
| Air | 28.96 |
| Nitrogen | 28.02 |
| Oxygen | 32.00 |
| Carbon Dioxide | 44.01 |
| Custom | User-defined |
Steps:
The specific gas constant is critical for:
Example 1: Calculate the specific gas constant for air using the molar mass method, with \( R = 8.31446261815324 \, \text{J/(mol·K)} \), output in J/(kg·K):
Results:
Example 2: Calculate the specific gas constant for a gas with \( C_p = 1005 \, \text{J/(kg·K)} \), \( C_v = 718 \, \text{J/(kg·K)} \), output in kJ/(kg·K):
Results:
Q: What is the specific gas constant?
A: It’s a gas-specific constant used in the ideal gas law (\( P = \rho R_{\text{specific}} T \)), relating pressure, density, and temperature.
Q: When to use the molar mass vs. specific heat method?
A: Use molar mass for known gases or mixtures with defined molar masses. Use specific heat for gases where \( C_p \) and \( C_v \) are measured, especially mixtures.
Q: Why must \( C_p > C_v \)?
A: Thermodynamics requires \( C_p > C_v \) because work is done by a gas at constant pressure, increasing its heat capacity.
Q: Is this calculator valid for real gases?
A: Yes, for low-density, high-temperature conditions where ideal gas assumptions hold. For high pressures, consider real gas corrections.
Q: Can I modify the universal gas constant?
A: Yes, the default is 8.31446261815324 J/(mol·K), but you can edit it for custom calculations.