 Home
Home
 Back
Back
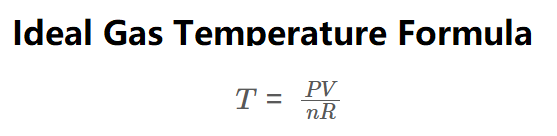
Definition: This calculator computes the temperature (\( T \)) of an ideal gas using the ideal gas law. As shown in the formula image above, temperature is calculated via:
\( T = \frac{PV}{nR} \)
Users input pressure, volume, and number of moles, selecting appropriate units. Results are displayed with 5 decimal places in Kelvin, Celsius, or Fahrenheit.Purpose: Essential for thermodynamics, physics, and engineering to determine gas temperature in applications like HVAC systems, gas storage, and laboratory experiments.
The calculator derives gas temperature from the ideal gas law (\( PV = nRT \)), rearranged as:
\( T = \frac{PV}{nR} \)
Where:Steps:
Gas temperature calculations are critical for:
Example 1: Calculate the temperature of 1 mol of gas at 1 atm pressure and 22.4 L volume, output in K and °C:
Results:
Example 2: Calculate the temperature of 0.5 mol of gas at 2 bar pressure and 0.01 m³ volume, output in K and °F:
Results:
Q: What is the ideal gas law?
A: The ideal gas law, \( PV = nRT \), relates pressure, volume, temperature, and moles of an ideal gas, assuming no intermolecular forces and elastic collisions.
Q: Why use Kelvin for calculations?
A: Kelvin ensures positive temperatures, aligning with the ideal gas law’s requirements. The calculator converts to °C or °F for convenience.
Q: Is this calculator valid for real gases?
A: Yes, for gases like air, nitrogen, or oxygen at high temperatures and low pressures, where ideal gas behavior is a good approximation.
Q: What if I don’t know the number of moles?
A: You can estimate moles using mass and molar mass or measure gas quantity experimentally.